Using mobile phase pH for method development – A case study with cannabinoids and heterocyclic compounds
A couple short anecdotes about how pH can help or hurt your chromatography
CHEMISTRY
Charles M. Nichols
4/22/20245 min read


One important aspect of method development when using reverse phase chromatography is to optimize the pH of the mobile phase. I did not learn this until I transitioned form LCMS to HPLC with non-MS detectors such as UV-VIS, fluorescence, RI, or ELSD. In general, the LCMS chemist does not think about pH when doing method development because co-eluting molecules can often be resolved in the m/z dimension. In contrast, non-MS detectors require baseline resolution to achieve accurate quantitation. In this regard, method development for HPLC requires a more thoughtful approach to optimize the mobile phase.
Sometimes, when peaks coelute, baseline resolution can be achieved by adjusting the gradient (either steepening or shallowing the gradient). However, for many molecules and mobile phase compositions, adjusting the gradient won’t suddenly achieve separation. When molecules co-elute, you need to investigate the nature of these molecules and try to exploit some of the physical differences. If the co-eluting molecules have different acid-base chemistry (i.e. one acid and one neutral; one base and one neutral; one acid and one base), it could be possible to achieve separation by adjusting the pH of the mobile phase.
Let’s investigate how pH can adjust the retention of a specific molecule by exploring an analytical HPLC panel of 16 endocannabinoids. In this example, the two compounds THCA and CBC co-elute. For this example, THCA contains an acidic proton, whereas the co-eluting molecule, CBC, does not contain a labile proton. First, let us consider that THCA which has a pKa value of 2.89. The pKa of CBC has not been measured, but it is estimated to be ~9.47. The pH of 5 mM ammonium formate with 0.1% formic acid is ~3.0, and this is very near the pKa of THCA. Adjusting the mobile phase by very slightly increasing the concentration of ammonium formate, we can increase the pH (Table 1). This can effect the retention of THCA to achieve separation.
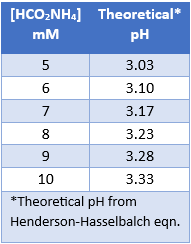
Table 1. Aqueous ammonium formate with 0.1% Formic Acid
As the pH of the solution is increased above the pKa of THCA, THCA dissociates a proton and becomes a negative ion. Ions are intrinsically more polar than neutral molecules. As C18 stationary phase is highly non-polar, the retention time of THCA is decreased, allowing baseline resolution from CBC, Figure 1. These chromatograms illustrate the power of exploiting pH for method development. This approach can also be used to adjust the retention time of basic compounds, although the opposite must be considered. For basic compounds, reducing the pH below the pKa of the conjugate base can ionize the base by appending a proton which will led to a reduction in analyte retention. Further trends can be inferred from the chromatograms in Figure 1. For example, the acid THCVA does not show a drastic effect from the pH adjustment. The early eluting compounds show less effect when adjusting the pH, so this approach likely wouldn’t work for an acid-neutral pair that elutes early. Furthermore, it is apparent that the neutral molecules show virtually no effect from the pH adjustment. Keep these observations in mind when considering a pH adjustment to improve your chromatography for a diverse panel of molecules.
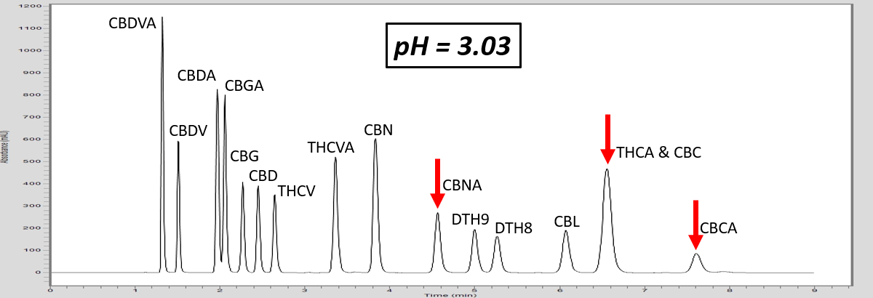
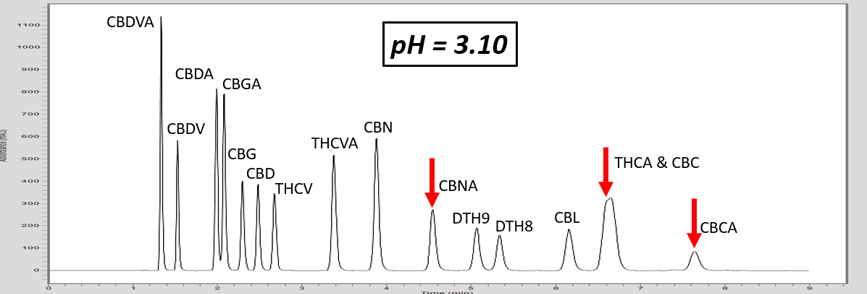
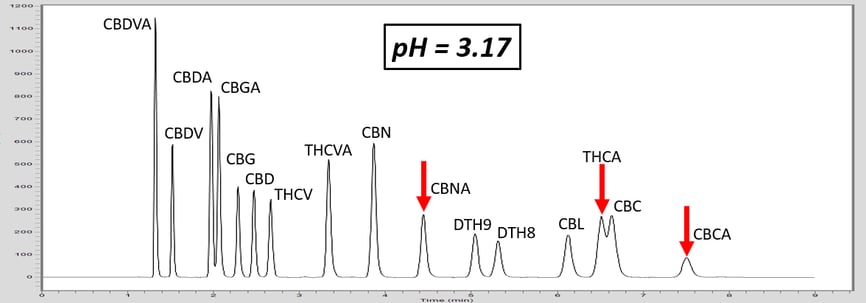
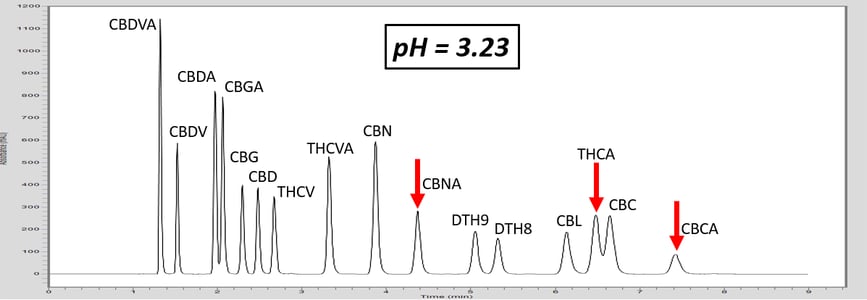
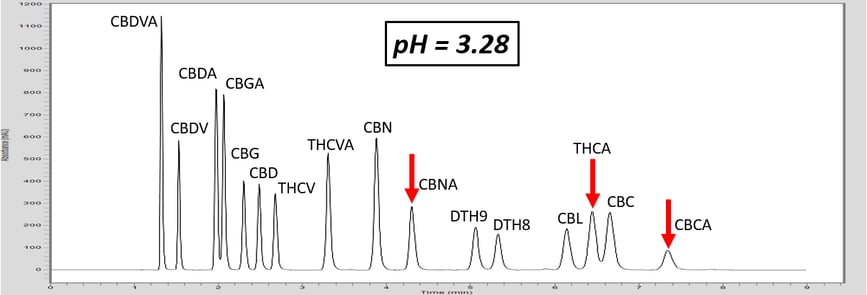
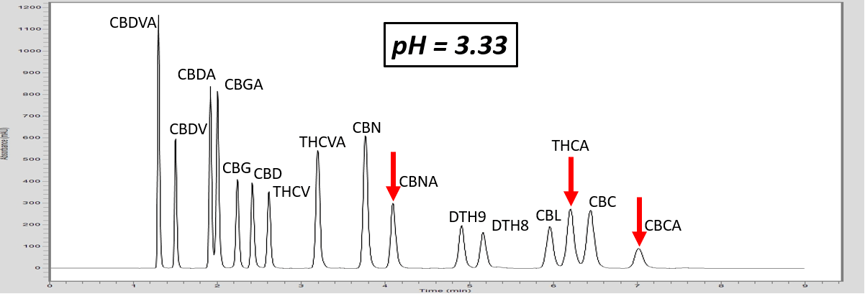
Figure 1. As the pH of the aqueous mobile phase is increased, the acidic endocannabinoids become ionized. This induces a reduction in retention of the acidic analyte, and allows for baseline resolution of THCA and CBC.
While the above example shows how pH can be exploited to improve chromatography, it can also cause issues. The following example illustrates how a mismatch in the pH of the sample diluent and mobile phase can lead to poor peak shape. In the example below, Figure 2, the samples were dissolved in diluent free of additives. This problem was intensified by the fact that the mobile phase did not have any buffering capacity (the only additive to the mobile phase was formic acid).
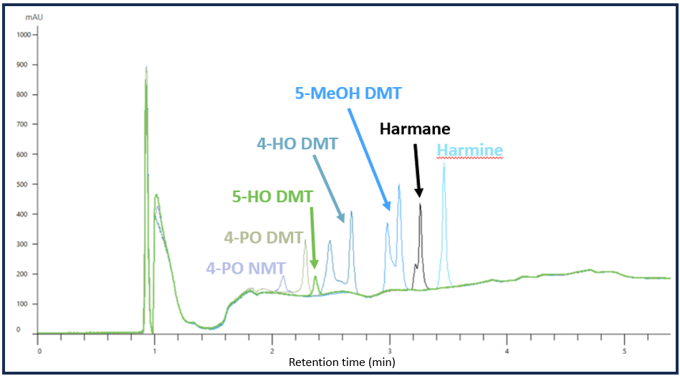
Figure 2. A chromatogram showing severe peak splitting from pH and buffer capacity issues with the sample diluent.
In this example, 4-OH DMT, 5-MeOH DMT, and Harmane show severe peak splitting because the sample diluent caused a drastic rise in the pH resulting in poor retention. This pH discrepancy upon injection likely forced some of the target analyte into an ionized form reducing their retention on the column. After reoptimizing the mobile phase and sample diluent, the peak shapes are drastically improved, Figure 3.
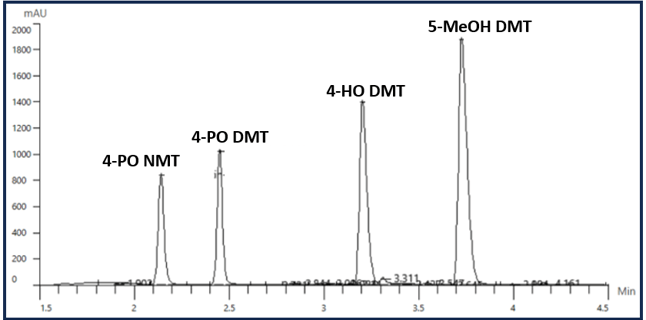
Figure 3. An improved separation from optimizing the sample diluent and mobile phase.
In summary, it is important to consider the effect that mobile phase and diluent pH might have on the retention of target analyte in an HPLC assay. pH can be a powerful tool for HPLC and LCMS method development, and the more tools you have the better you will be at building robust liquid chromatography measurements. This post only serves as anecdote for two examples where pH played a critical role in the selectivity and peak shape. There are many other posts and publications online that go into more depth about the importance of pKa and pH for mobile phase determination. Discussions of buffers and other mobile phase additives can warrant an entire chapter in a chromatography textbook. Please reach out if you have any questions about the role of pKa and pH in LCMS method development, and I would be happy to share resources or discuss the philosophy in more detail.
